What is a Continuous Spectrum?

The term continuous spectrum is mostly found in physics and mainly involves light and colors found therein. Spectra also help us understand how atoms absorb different light energies to provide the color we see. There are two main types of spectra. Continuous and line spectra and while these are generally different, it is possible to have both of them. Continuous spectrum can be learnt vividly in the context of light and electromagnetic spectrum. Here is a brief definition and description of these spectra as well as their differences:
Continuous Spectrum Definition
Those who have seen a rainbow have probably seen a continuous spectrum as it is agreed that the sun’s light contain this kind of spectrum although you will learn that this is not so all the time. When white light is passed through a prism, it produces a rainbow of colors because of the different wavelengths that refract (bend) at different angles when they go through the prism.
If the rainbow that is produced has all the colors from red to violet, and has no gaps between them, then it is referred to as a continuous spectrum. A beam of pure white light attainable is some laboratories often contain this spectrum. It is also possible to obtain continuous spectra when you heat up different materials until they glow.
The correct continuous spectrum definition therefore exhibits when, within a given limit, all the wavelengths are present.
Learn More About Continuous Spectrum
Doing more homework on continuous spectrum? Don’t worry – we have you covered with even more insights and knowledge on this fascinating topic.
How Is A Continuous Spectrum Produced?
When Newton did his famous experiment with a prism and sunlight, he noted that the Sun produced a “rainbow” of colors. This is a continuous spectrum. However if he had been able to produce a more detailed spectrum he would have noticed some subtleties on this continuous spectrum. So, light from the Sun, and any star, produces a continuous spectrum.
We also get a continuum spectrum from a hot solid, so for example the light produced by incandescent light bulbs is a continuum spectrum. These kinds of bulbs give off light by a very thin coil of metal, the filament, (usually tungsten) getting extremely hot from having an electric current passed through it. When the filament gets to thousands of degrees, it gives off light (source).
Continuous Spectra Vs. Line Spectra
When continuous spectrum, like in a rainbow, comes from white light, line spectrum is evident in colored compounds. Light spectrum only has a few wavelengths (not all) or lines. Atoms tend to absorb some wavelengths when electromagnetic radiation is passed through them which display only a few narrow absorption lines when recorded.
The major difference between these two is that continuous spectra has all the wavelengths while line spectrum only contains some of the wavelengths. Line spectrum can also be generated in emission and absorption spectrum while continuous spectrum occurs when both absorption and emission spectra of a single species are put together. In other words, line spectrum can be in either emission spectrum or absorption spectrum.

The Chemistry Of Continuous Spectrum
In order to fully understand continuous spectrum chemistry, it is important to discern electromagnetic spectrum. White light is a continuous spectrum but this is only part of a larger electromagnetic spectrum that contains radio waves, infrared rays, microwaves, ultraviolet, gamma rays and x-rays. However, no single object in the universe is known to send out waves through the wider electromagnetic spectrum which means you cannot find continuous electromagnetic spectra.
Even with the sun’s light which is thought to be continuous since we see rainbows after it rains. There are often gaps where nothing is visible when the light is analyzed in detail through a spectrometer. A truly continuous spectrum should not have any gaps and identifying gaps in the sun’s light is how scientists know what it is made of. The absorption gaps detail other elements including hydrogen and helium.
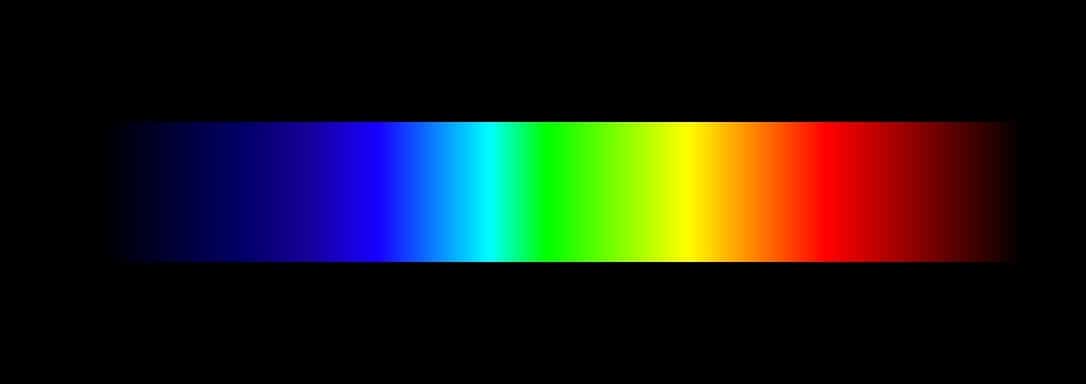
Example
Rainbow is generally accepted as an example as it details all the colors (wavelengths) from red to violet without any gaps. However, the sun’s light is not a perfect example of continuous spectrum as it contains absorption gaps therein. A more ideal continuous spectrum example can be shown when you pass white light through a prism in well set lab environments. Other physicists have also shown that heating up objects to the point which they glow can produce the spectrum. This is because atoms emit white light at glowing point and have thus given away all the energy absorbed.
In order to generate complete continuous spectra, we need to put together absorption and emission spectra. An emission spectrum is the exact opposite of an absorption spectrum. When an absorption spectrum shows a few wavelengths with some particular colors missing. An emission spectrum only shows the colors missing in an absorption spectrum. Thus, combining the two would give you all the wavelengths that form a continuous spectrum.
Want to Dive Even Deeper into the Science?
Learn more about the science of the continuous spectrum here and let your creativity run wild.
